Behind the Curtain: Unveiling Misconduct in MDMA Psychotherapy Trials
Content Warning: Descriptions of Suicidality, Patient Abuse, and Sexual Violence
PART ONE… Raising Red Flags: Petitioners Challenge MAPS’ Ethical Integrity
Lykos Therapeutics’ new drug application for MDMA (street names: Adam/Ecstasy/Molly) may soon be approved by the Food and Drug Administration (FDA) as a pharmacological adjunct to various psychotherapies for the treatment of post-traumatic stress disorder (PTSD). However, a recently filed petition addressed to the FDA has raised significant concerns related to the conduct of the for-profit Lykos and its parent non-profit research organization known as MAPS within and outside the context of their FDA-regulated clinical trials examining MDMA-assisted psychotherapy (MDMA-AP).
Released on April 10, the petition implores the FDA to convene an “extended open public hearing” so that various individuals (including former Lykos/MAPS trial participants) may express their evidence-based “concern[s]” regarding the clinical research conducted by Lykos/MAPS researchers.
Written by an interdisciplinary group of researchers, the petition alleges that Lykos/MAPS staff, researchers, and therapists operate within a “culture of silence [and] fear” as it pertains to addressing “pervasive issues” related to the organizations’ operations as well as the documented shortcomings concerning their clinical research.
To wit, the petitioners detail an alleged incident in which a Lykos/MAPS trial subject engaged in suicidal behaviour during an MDMA-AP trial session — an event which would likely constitute an “unexpected serious adverse event”. This serious adverse event, the petition alleges, was not reported properly after study therapists allegedly consulted with Richard Doblin, Ph.D. (MAPS Founder/President and Lykos Board Director).
Back in December 2023, Lykos Therapeutics (formerly MAPS Public Benefit Corporation) submitted a new drug application (NDA) to the FDA which is currently undergoing a prioritized review given MDMA’s designation as a “breakthrough therapy”. MDMA advocates have celebrated Lykos’ NDA submission with the hopes that this investigational form of drug-assisted psychotherapy will outperform currently available treatment options. Yet, there’s more to the story than meets the eye.
Given that Lykos’ NDA submission represents the first opportunity for the FDA to consider approving an investigational psychedelic drug paired in combination with psychotherapy, the unprecedented nature of this drug application cannot be overstated. Furthermore, twenty-five years have elapsed since the FDA last approved a pharmacological treatment for PTSD (in this case, Zoloft/sertraline), signifying that this ‘prioritized review process’ will be a generation-defining decision by the FDA.
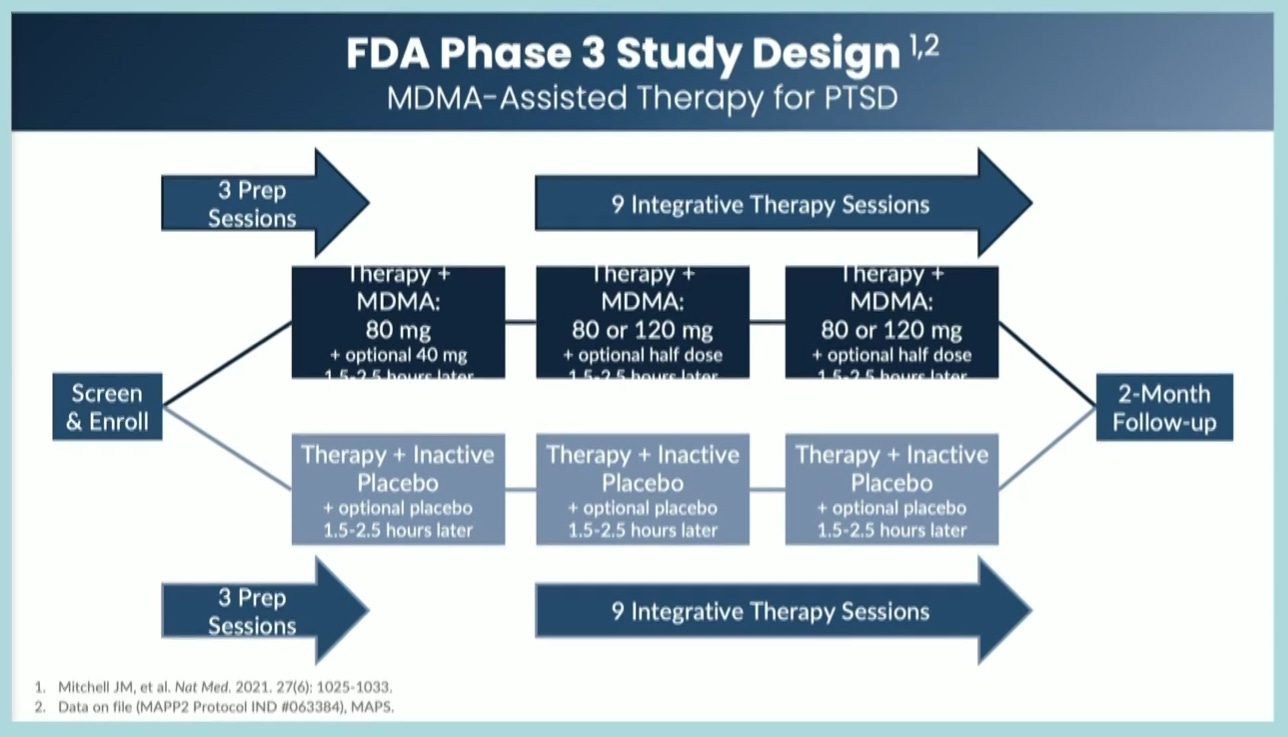
Lykos’ NDA indicates that the non-standardized psychotherapeutic component designed to be paired with MDMA is far from conventional. Endorsing the incorporation of “elements” of up to thirteen “psychotherapeutic approaches” within preparatory, treatment, and follow-up ‘integration’ sessions, the MAPS Treatment Manual proposes that the mechanism of action yielding impressive trial outcomes is a pseudoscientific concept known as the “inner healing intelligence”, a notion which will be discussed further in this blog post. Beyond this, the non-negligible portion of these “psychotherapeutic approaches” have not been validated by a robust body of peer-reviewed clinical trial publications.
Beyond the issues described above, the petition calls attention to an alleged institution-wide “pattern of systematic and deliberate omission of adverse events from the public record” by Lykos/MAPS researchers. The petition also addresses concerns related to known incidents of increased suicidality in MDMA trial subjects as well as “boundary violations” effectuated (in part) by Lykos/MAPS’ incorporation of physical touch into the treatment protocol.
Apart from that, the petition states that Lykos/MAPS representatives “may be” actively “skewing public perception” regarding the overall risk profile and therapeutic efficacy of MDMA-AP. By way of example, the petition describes how MAPS has employed former Lykos/MAPS trial subjects for “public relations and political lobbying campaigns”.
It should be noted that MAPS founder Richard Doblin frequently states that MAPS does not conduct ‘science’, but rather ‘political science’. As he commonly puts it, “We don't do science. We do political science."
An anonymous whistleblower identified in the petition as a former employee of MAPS Public Benefit Corporation alleged that Lykos/MAPS investigators promptly contacted Richard Doblin if an adverse event occurred within a MAPS-sponsored clinical trial so that he could “determine” if the event(s) in question “should be reported” to the relevant authorities, including the FDA and Lykos/MAPS’ institutional review board (IRB). It should be noted that reporting these events is required by those regulatory bodies. According to the petition, Doblin allegedly replied to these investigators’ requests with “justifications” for the omission of adverse event(s) from reports/filings with these regulatory authorities.
![Quote from Doblin in 2020: "[S]o, the legal system has never been the guide for me, you know? It's a guide about what people think is wrong and right. I have to consider it, but I've never thought that it's (like) the final word on things. But a lot of people are, uhm, worried about that. So, that's... that's really difficult" Quote from Doblin in 2020: "[S]o, the legal system has never been the guide for me, you know? It's a guide about what people think is wrong and right. I have to consider it, but I've never thought that it's (like) the final word on things. But a lot of people are, uhm, worried about that. So, that's... that's really difficult"](https://substackcdn.com/image/fetch/$s_!m-1B!,w_1456,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F60cd8b78-2f07-4df6-9fb1-ed56b5975e24_650x650)
The petition states that in the immediate aftermath of the above-described incident involving the Lykos/MAPS trial subject allegedly engaging in suicidal behaviour during an MDMA-AP treatment session, Doblin allegedly advised the subject’s therapists to “not report the incident”. The petition clarifies that it was allegedly Doblin’s professional belief that the act(s) of suicidal behaviour during the trial session was/were related to the subject’s “personal circumstances” as opposed to investigational drug (MDMA) or the adjunctive, non-standardized form of psychotherapy.
Furthermore, the petition documents how numerous trial subjects in MAPS-sponsored clinical trials have “attribut[ed]” their experiences of exacerbated suicidal ideation/behaviour as a direct result of receiving this novel medical intervention. Despite this, these MDMA-associated events of exacerbated suicidal ideation/behaviour were not reported as such in the published clinical literature nor the MAPS’ Investigator’s Brochure for MDMA.
The revelations surrounding Lykos/MAPS’ research practices (as detailed in the petition) cast a critical spotlight on the intricate history of MAPS, a pioneer in the field of psychedelic research. Founded in 1986 by Richard Doblin as a counter-response to the federal prohibition of MDMA, the Multidisciplinary Association for Psychedelic Studies (MAPS) emerged as a non-profit pharmaceutical research organization seeking to explore the therapeutic potential of psychedelic drugs (namely MDMA) as well as cannabis.
In 2014, MAPS established MAPS Public Benefit Corporation (MAPS-PBC) as a wholly-owned for-profit subsidiary, but the for-profit changed its name to “Lykos Therapeutics” following the acquisition of approximately $100M in investments reported in January 2024. These investments originated from three venture capitalist firms (KittyHawk Ventures, Vine Ventures, True Ventures), three non-profits (Steven & Alexandra Cohen Foundation, Unlikely Collaborators Foundation, Joe & Sandy Samberg Foundation), two investment firms (Bail Capital and Satori Neuro), and one biotech company (Eir Therapeutics). As of the present moment, MAPS holds the largest stake in Lykos (albeit a minority stake), signaling a significant evolution in the for-profit’s sources of funding.
The main distinctions between MAPS and Lykos in terms of their clinical research can be adequately summarized in the following terms: Whereas the relevant trial publications in the journals Psychopharmacology and Nature Medicine indicate that MAPS “sponsored” and “fully funded” these phase-two and phase-three MDMA studies, Lykos/MAPS-PBC is credited as the “trial organizer” for these aforementioned clinical trials examining MDMA-AP.
PART TWO… Corroborating Claims: My Investigations Support the Petition’s Allegations
Given that I have spent half of my life dealing with disordered post-traumatic stress originating from adverse childhood experiences, I started a fundraiser for MAPS three years ago to raise money for their cause. Indeed, I supported their research at the time because I desired to benefit from MDMA-AP and believed in their vision of a world with less suffering. Make no mistake about it — I considered it to be in my best interests to champion those who were investigating a promising treatment modality for PTSD. However, last year, I called for the resignation of MAPS’ founder Richard Doblin via a podcast and a separate petition.
The inspiration behind this choice came after I realized that Doblin was engaging in acts that I have previously described as “abusing the public’s trust”. By way of example, I have specifically cited his proposal to enroll up to 400 war-torn, traumatized Ukrainians (housed in refugee centers) into placebo-controlled MDMA studies under the auspices of a “humanitarian project” sponsored by MAPS.
To be specific, Doblin has proposed conducting a clinical trial located at “some sort of stable refugee center or something” wherein “several” psychotherapists could administer a singular session of MDMA-assisted (and placebo-assisted) group psychotherapy to upwards of 100 people at a time. The motivation behind such a clinical trial would be to determine if MDMA-assisted group psychotherapy has any efficacy in prophylactically treating post-traumatic stress to prevent its development into chronic PTSD. Although Doblin has acknowledged why long-term follow-up research would be “challeng[ing]”, he claims that such a clinical trial “could” assist the nation of Ukraine in “mov[ing] forward” from a war that has displaced millions.
Moreover, Doblin has stated that the relative lack of Ukrainian therapists would make it difficult to find adequately trained professionals to “lead” these experimental drug-assisted group psychotherapy sessions. To overcome this hurdle, Doblin has proposed the implementation of yet-to-be-determined “local healers” who do not have appropriate accreditation to manage clinical trial sessions and, thus, are not qualified to administer this investigational form of drug-assisted group psychotherapy.
It should be noted that Articles 6 and 8 of the Nuremberg Code state that:
“The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment [...] The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct or engage in the experiment.”
Although Doblin told the Boston Psychedelic Research Group that the “humanitarian projects” currently being planned by MAPS would be “riskier” than Lykos/MAPS’ previous clinical trials, he followed up this statement by asserting that they represent “a risk we [MAPS] must take”. By way of contrast, the principal investigator of a Bronx-based MAPS-sponsored clinical trial has stated that she “would not conduct such a study now or maybe even ever [...]”, signifying that dissent exists within the ranks of MAPS-affiliated researchers as it pertains to the organization’s endeavors.
Beyond the concerns listed above, I felt a moral/ethical obligation to call for Doblin’s resignation given that he has claimed to have attempted to persuade Lykos/MAPS to be less risk-averse regarding their clinical research. Perhaps the straw that broke the camel’s back was that I became convinced that Doblin has repeatedly encouraged people without appropriate licensure to perform MDMA-assisted psychotherapy (MDMA-AP) upon their “friends”.
People have disagreed with this evidence-based perspective, but Doblin’s statements speak for themselves. As the saying goes, res ipsa loquitur. In case my audience needs reminding, practicing psychotherapy without a license (regardless of whether a controlled substance is administered) is a criminal offense in many jurisdictions.
“For the people that are suffering […] you could offer to sit with them […] you could learn from our treatment manual [about] how to help them” - Doblin on the Camp Mystic podcast
Back in 2020, Doblin appeared on the HealthyGamerGG podcast where he discussed various legal means for people to access psychedelic drugs. Immediately following this, he noted that MAPS offers various “training materials” including the MAPS Treatment Manual. He clarified that this publication is freely available on the MAPS website, adding that the manual “explains how our therapeutic approach is done and you can practice it on your own with your friends [...]”.
Near the end of his presentation at the 2016 Horizons Conference, Doblin shared his belief that MDMA is “inherently therapeutic on its own”, supplementing this hyped assertion with unverifiable anecdotes of Burning Man attendees attesting to this. After reminding his audience that the MAPS Treatment Manual is obtainable without any paywalls, he asserted that trustworthy “friends” can offer a therapeutic alliance “at virtually no cost”. He concluded this statement by clarifying that if this option is not satisfactory for various individuals, they could receive psychedelic-assisted psychotherapy in “clinic[al] settings” by “trained therapists”.
Months before Doblin’s presentation at Horizons, Doblin answered Duncan Trussell’s question of whether users of psychedelic drugs can build “therapeutic alliances” with friends who also consume these substances. Specifically, Trussell noted the widespread lack of affordable access to psychotherapists, yet shared his perspective that many people consume psychedelics in group settings. Trussell followed up by asking Doblin whether people can “create [a] therapeutic alliance with friends” in this specific context, to which Doblin stated, “Yeah! Yes! Yes! Totally!”.
Aware of the risks associated with violating the Nuremberg Code and practicing drug-assisted psychotherapy without appropriate licensure, I felt called to inform the public of the risks associated with the ideas Doblin has promoted.
To substantiate a portion of the petitioners’ claims, I will outline several instances of misconduct and malpractice within Lykos/MAPS research practices. Moreover, I will examine the implications of this evidence as it relates to the credibility and safety of Lykos/MAPS’ particular approach to MDMA-AP as a therapeutic intervention.
The following sections examine the veracity of a portion of the petition’s claims, exploring the documented instances of institutional malfeasance/misconduct at Lykos/MAPS. For reference, the majority of incidents briefly described in the sections below have been documented in full detail within my pre-print entitled “Omission Of Serious Adverse Event(s) Within MAPS-Sponsored Clinical Trial Publications Examining MDMA-Assisted Psychotherapy For PTSD”.



